Developing a Pharma CRM requires a meticulous focus on security and regulatory compliance to safeguard sensitive data, maintain user trust, and meet the stringent requirements of the pharmaceutical industry. Ensuring that systems adhere to global and regional regulations like HIPAA, GDPR, and the Sunshine Act is paramount. Below are best practices that developers should follow to create secure and compliant Pharma CRM tools.
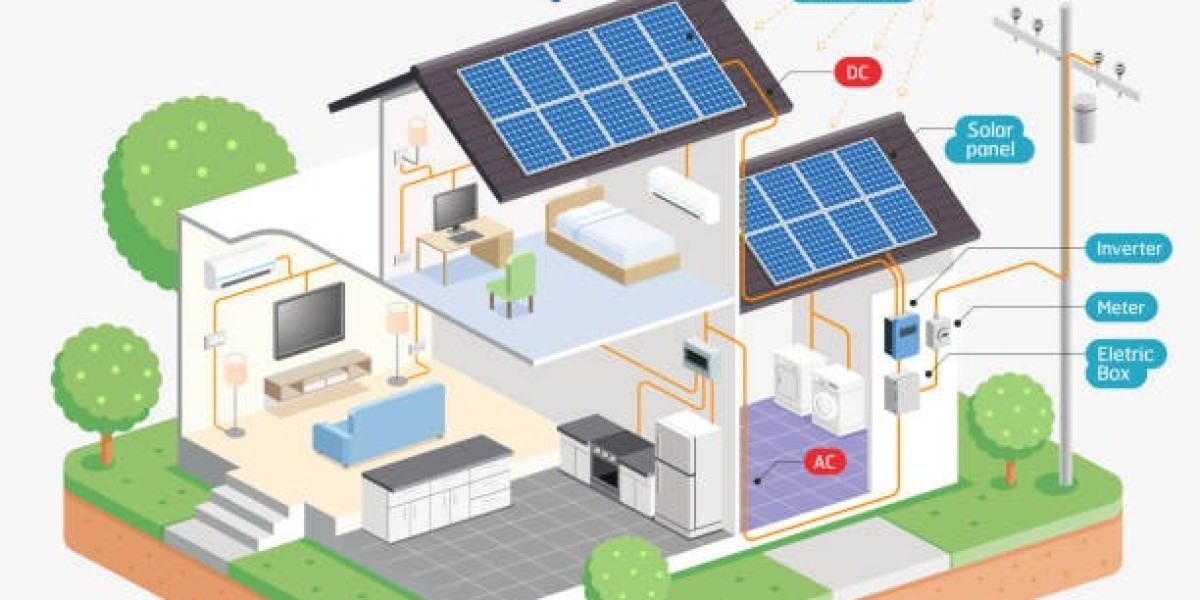
Pharma CRM platforms should employ robust data security measures, including end-to-end encryption (e.g., AES-256) to protect sensitive information both at rest and in transit. Developers should implement role-based access control (RBAC) to restrict data access based on user roles, along with multifactor authentication (MFA) for enhanced login security. To meet compliance needs, audit trails must track every interaction and modification within the system, ensuring that all actions are logged for regulatory reporting and inspections.
To maintain data privacy and adhere to regulations such as GDPR and HIPAA, developers need to incorporate mechanisms for consent management, allowing users to control their data. Data minimization principles should also be followed, collecting only the information necessary for business operations. Privacy by design should guide the systems architecture, ensuring that compliance is baked into the development process rather than treated as an afterthought.
Regulatory compliance is further supported by regular validation testing. This includes system validation to meet standards like 21 CFR Part 11 for electronic records and electronic signatures. Developers should conduct periodic penetration testing and vulnerability assessments to identify and mitigate security risks proactively. Additionally, systems should provide automated tools for regulatory reporting, such as Sunshine Act transparency reports or GDPR data access logs.
For field representatives and mobile users, offline functionality must adhere to the same compliance standards as online systems. This includes encrypting local data and synchronizing securely with cloud servers when connectivity is restored. Developers must also address challenges like secure sample tracking and product distribution, ensuring that all data related to sample handouts or promotional activities is fully traceable.
Effective data integration is crucial in Pharma CRM development, as the platforms often connect with external systems like electronic health records (EHRs), marketing automation tools, and third-party data providers. All integrations should use secure APIs, with strong authentication protocols and data validation processes to prevent unauthorized access or data corruption.
In summary, Pharma CRM tools must incorporate robust encryption, role-based access control, audit trails, and regulatory reporting to ensure security and compliance. Privacy by design, validation testing, and secure data integrations further support adherence to global regulations. By following these best practices, developers can build systems that are both secure and compliant while meeting the unique needs of the pharmaceutical industry.