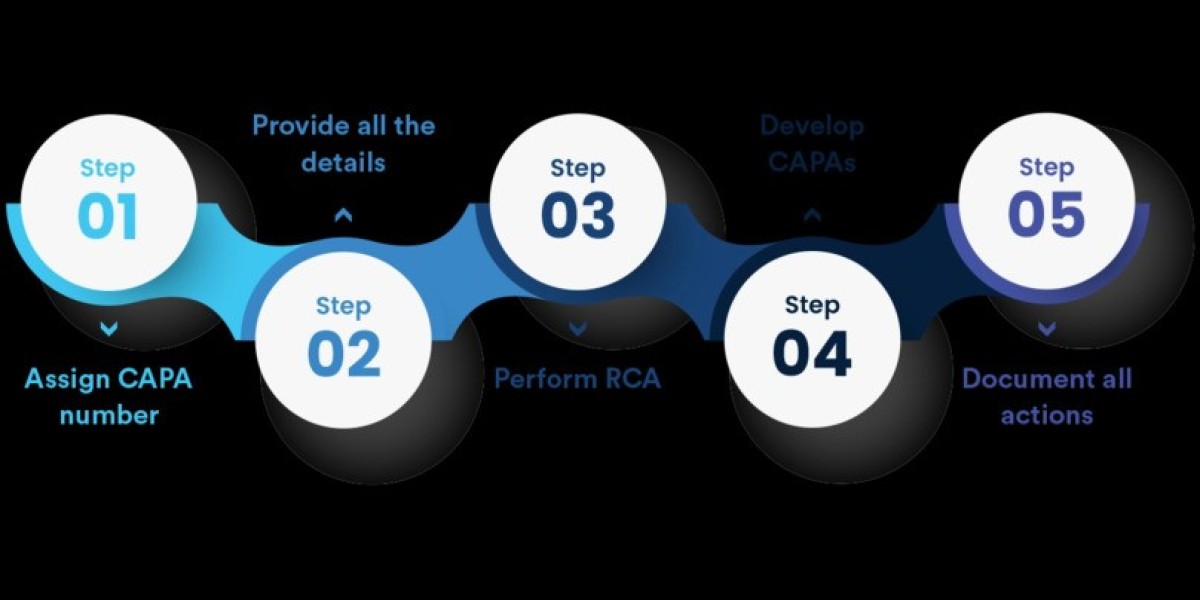
Understanding NC CAPA and Its Importance
NC CAPA
Effective NC CAPA processes help prevent the recurrence of defects, ensuring consistent product quality and regulatory compliance.
The Critical Role of Documentation in NC CAPA
Documentation serves as the backbone of the NC CAPA process. It provides a detailed record of non-conformances, investigations, root cause analyses, and the actions taken to address and prevent future issues. Proper documentation ensures transparency, accountability, and traceability, which are essential for successful CAPA implementation.
Documentation in CAPA for Manufacturing
CAPA in Manufacturing: Ensuring Quality and Efficiency
CAPA in the manufacturing sector, is integral to maintaining product quality and operational efficiency. Detailed documentation helps manufacturers track non-conformances, analyze trends, and implement effective corrective and preventive measures.
How Documentation Enhances CAPA in Manufacturing
Accurate documentation allows manufacturing companies to systematically address non-conformances. By maintaining comprehensive records, manufacturers can identify recurring issues, streamline processes, and ensure that corrective actions are effectively implemented and monitored.
The Unique Challenges of CAPA in the Pharmaceutical Industry
The pharmaceutical industry faces stringent regulatory requirements and high standards for product quality. Managing CAPA in the pharmaceutical industry involves navigating complex compliance landscapes to ensure that all corrective and preventive actions meet regulatory expectations. CAPA processes must be meticulously documented to comply with regulations and ensure patient safety, making effective documentation crucial for success in this highly regulated sector.
Documentation Strategies for Effective CAPA in the Pharmaceutical Industry
Pharmaceutical companies must adopt robust documentation practices to manage CAPA in the pharmaceutical industry effectively. This includes maintaining detailed records of non-conformances, conducting thorough investigations, performing root cause analyses, and implementing preventive measures. Comprehensive documentation not only ensures regulatory compliance but also enhances the overall quality management system, enabling pharmaceutical companies to address issues proactively and maintain the highest standards of product quality and safety.
CAPA for Medical Devices: The Role of Documentation
Ensuring Compliance in Medical Device Manufacturing
Medical device manufacturers must adhere to strict regulatory standards to ensure the safety and efficacy of their products. CAPA Medical Device processes, supported by thorough documentation, are essential for maintaining compliance and addressing any non-conformances that arise. Implementing a robust CAPA Medical Device system ensures that manufacturers can systematically identify and rectify issues, thereby safeguarding product quality and patient safety.
Documentation Best Practices for CAPA Medical Device in Software for Manufacturing
For companies involved in medical device software for manufacturing, precise documentation is critical. Effective CAPA Medical Device practices include detailed records of software defects, validation processes, corrective actions, and preventive measures. Proper documentation ensures that all aspects of the CAPA Medical Device process are transparent and traceable, facilitating regulatory audits and continuous improvement. By integrating CAPA Medical Device documentation best practices, manufacturers can enhance their ability to respond to software-related non-conformances swiftly and efficiently, ensuring ongoing compliance and product reliability.
Integrating Documentation with Advanced Quality Management Systems
Advantages of Utilizing Advanced Quality Management Systems for NC CAPA
Modern quality management systems offer integrated solutions for managing NC CAPA processes. These systems provide centralized documentation, streamlined workflows, and real-time tracking, enhancing the efficiency and effectiveness of CAPA activities.
Leveraging Technology for Superior Documentation in CAPA Processes
Advanced quality management systems leverage technology to automate documentation tasks, reduce errors, and ensure consistency. By integrating NC CAPA documentation into a unified platform, organizations can enhance collaboration, improve data accuracy, and accelerate the resolution of non-conformances.
Overcoming Common Documentation Challenges in CAPA
Identifying and Addressing Documentation Gaps
One of the primary challenges in CAPA documentation is ensuring completeness and accuracy. Organizations must implement robust documentation practices to avoid gaps that can hinder the effectiveness of CAPA processes.
Strategies to Enhance Documentation Quality in CAPA Processes
To overcome documentation challenges, companies should adopt standardized templates, provide comprehensive training, and utilize advanced quality management systems. These strategies help ensure that all necessary information is captured accurately and consistently, facilitating successful CAPA outcomes.
The Impact of Effective Documentation on Regulatory Compliance
Meeting Regulatory Requirements Through Documentation
Regulatory bodies require detailed documentation of NC CAPA processes to ensure that organizations maintain high standards of quality and safety. Effective documentation demonstrates compliance and readiness for regulatory audits.
How Documentation Supports Regulatory Audits and Inspections
During regulatory audits, comprehensive documentation provides evidence of a company's commitment to quality and continuous improvement. Well-maintained records of non-conformances, investigations, and CAPA actions are critical for passing audits and avoiding penalties.
Best Practices for Documentation in NC CAPA Success
Establishing a Documentation Framework for CAPA
A robust documentation framework is essential for managing NC CAPA processes effectively. This includes defining clear documentation standards, establishing standardized procedures, and ensuring consistent record-keeping practices across the organization.
Continuous Improvement Through Documentation in CAPA Processes
Documentation should not be static; it must evolve with the organization's needs and regulatory requirements. Regularly reviewing and updating documentation practices ensures that CAPA processes remain effective and aligned with industry best practices.
Conclusion
In the rapidly evolving landscape of 2024, businesses in regulated industries must prioritize effective NC CAPA processes to stay competitive and compliant. ComplianceQuest offers a comprehensive quality management system that integrates advanced documentation capabilities, streamlining NC CAPA activities and ensuring regulatory compliance. By leveraging ComplianceQuest, organizations can enhance their CAPA processes, reduce non-conformances, and drive continuous improvement, positioning themselves for sustained success in a demanding market.