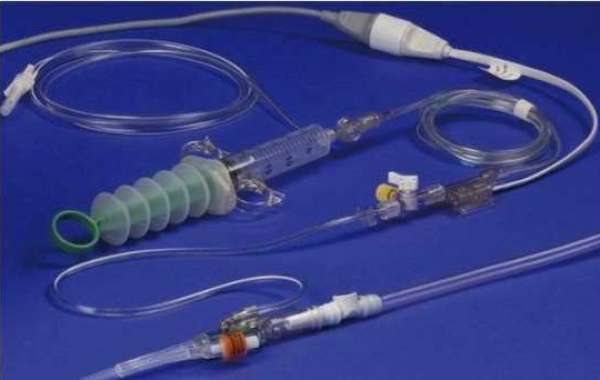
Intra-abdominal pressure measurement devices are medical devices used to measure intra-abdominal pressure in critically ill patients suffering from intra-abdominal hypertension. The devices are typically used in intensive care units to monitor patients at risk of developing complications associated with increased pressure in the abdomen, such as compromised circulation and organ dysfunction.
The global intra-abdominal pressure measurement devices market is estimated to be valued at US$ 106.7 Mn in 2023 and is expected to exhibit a CAGR of 16% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Dynamics:
Rising Cases of Intra-abdominal Hypertension (driver from heading): Intra-abdominal hypertension is a pathophysiological condition commonly seen in critically ill patients, including those with gastrointestinal complications, organ/tissue injuries, sepsis, severe burns, pancreatitis, and other conditions. The increasing prevalence of these conditions is driving the demand for intra-abdominal pressure measurement devices globally. According to a study published in the World Journal of Emergency Surgery in 2019, intra-abdominal hypertension affects approximately 60% of patients in the intensive care unit.
Another key driver boosting the growth of the intra-abdominal pressure measurement devices market is technological advancements. Manufacturers are developing more user-friendly and precise devices to monitor intra-abdominal pressure. For instance, in January 2022, Centurion Medical Products launched the updated version of its FoleyCatheter with automatic pressure measurement capabilities. Such innovative products are increasing the adoption of these devices among healthcare providers.
SWOT Analysis:
Strength: Intra Abdominal Pressure Measurement Devices Market has strong product portfolio with advanced medical devices that helps medical professionals to measure intra abdominal pressure accurately and efficiently. With innovative technologies, these devices provide reliable measurements to make clinical decisions. Presence of key players with global footprint helps in widespread availability of these devices.
Weakness: Intra Abdominal Pressure Measurement Devices Market depends on capital intensive research and development for continuous innovations. Stringent regulatory approvals and certification process increases costs of these medical devices. Lack of skilled professionals in developing countries hinders efficient use of advanced devices.
Opportunity: Rising prevalence of abdominal diseases like abdominal compartment syndrome drives the demand for intra abdominal pressure monitoring. Growing number of surgeries performed worldwide creates need for these devices. Developing healthcare infrastructure in emerging nations presents new opportunities.
Threats: availability of low cost alternatives limits the prices of branded devices. Economic slowdowns impact the healthcare expenses of people as well as hospitals. Stringent regulations on approval and adoption of new medical technologies is a challenge.
Key Takeaways:
The global Intra Abdominal Pressure Measurement Devices market is expected to witness high growth over the forecast period of 2023 to 2030.Regionally North America dominates the market currently due to presence of major players and advanced healthcare facilities. The hospitals segment accounts for the largest share due to high demand for continuous monitoring devices in intensive care units.
Regional analysis:
Asia Pacific region is expected to grow at the fastest pace during the forecast period. Rising medical tourism and government investments in healthcare sector of developing countries like India and China boost the demand. Large patient pool and growing awareness regarding abdominal pressure monitoring contribute to market growth in the region.
Key players:
Key players operating in the Intra Abdominal Pressure Measurement Devices market are CR. Bard, Inc. (Becton, Dickinson, and Company), ConvaTec Group PLC, Stryker Corporation, Biometrix Ltd. (Degania Silicone, Ltd.), Centurion Medical Products (Medline Industries, Inc.), and Potrero Medical