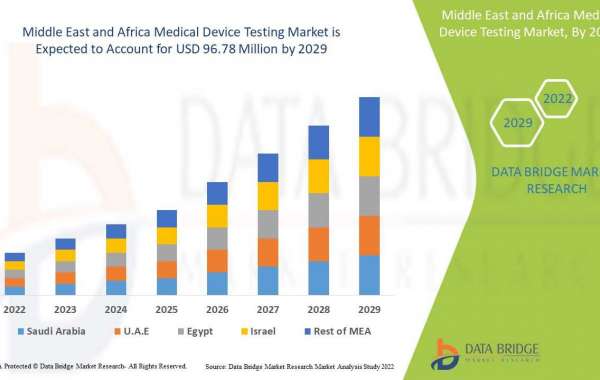
Middle East Africa medical device testing market is expected to grow in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 8.5% in the forecast period of 2022 to 2029 and is expected to reach USD 96.78 million by 2029 from USD 52.26 million in 2021.
Medical device testing is the process of demonstrating that the device is reliably and safely perform in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity and chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This usually include dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market such as inspection services, certification services and among others
View Detailed Report@ https://www.databridgemarketresearch.com/reports/middle-east-and-africa-medical-device-testing-market
Middle East and Africa Medical Device Testing Market Scope
Middle East and Africa medical device testing market is segmented into service type, testing type, phase, sourcing type, device class and product. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service Type
- Testing services
- Inspection services
- Certification services
On the basis of service type, the Middle East and Africa medical device testing market is segmented into testing services, inspection services and certification services.
Testing Type
- Physical testing
- Chemical/biological testing
- Cybersecurity testing
- Microbiology and sterility testing
- Others
On the basis of testing type, the Middle East and Africa medical device testing market is segmented into physical testing, chemical/biological testing, cybersecurity testing, microbiology and sterility testing and others.
Phase
- Preclinical
- Clinical
On the basis of phase, the Middle East and Africa medical device testing market is segmented into preclinical and clinical.
Sourcing Type
- Outsourced
- In-house
On the basis of sourcing type, the Middle East and Africa medical device testing market is segmented into in-house and outsourced.
Device Class
- Class I
- Class II
- Class III
On the basis of device class, the Middle East and Africa medical device testing market is segmented into class I, class II and class III.
Product
- Active implant medical device
- Active medical device
- Non-active medical device
- In-vitro diagnostics medical device
- Opthalmic medical device
- Orthopedic and dental medical device
- Vascular medical device
- Others
Browse More Reports:
https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
https://www.databridgemarketresearch.com/reports/europe-medical-device-testing-market
https://www.databridgemarketresearch.com/reports/asia-pacific-medical-device-testing-market
About Data Bridge Market Research:
An absolute way to forecast what future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavours to provide appropriate solutions to the complex business challenges and initiates an effortless decisionmaking process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475