Acceleration in technology development in the healthcare sector has tremendously increased in last few years. Advancement in technology of medical devices supports painless and uncomplicated treatment during disease management. Moreover, innovation and upgrading in medical devices assist in disease diagnosis's precise and rapid results. In addition, innovation in medical devices provides the cost-effectiveness of technology-based therapeutic tools during disease treatment.
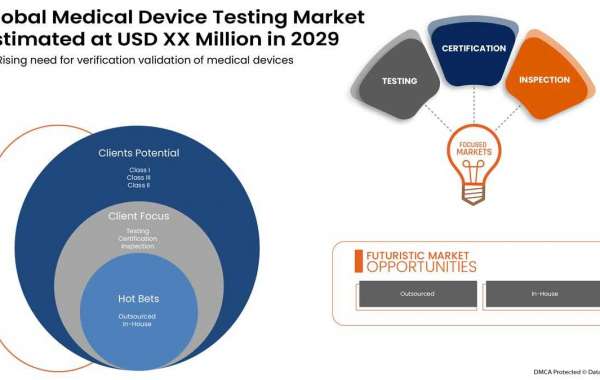
The rising need for verification validation of medical devices across the world will drive the market's growth rate
Medical devices are also becoming smaller and more complex in design using advanced, engineered plastics. This makes the process of validation and verification (VV) all the more important. This results in better repeatability, fewer mistakes, less rework and redesign, faster time to market, improved competitiveness, and lower production costs. Additionally, increasing standards and regulations about medical device validation and verification services are anticipated to drive the growth of the medical device testing market.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
Market Development
- In April 2021, TÜV SÜD announced that it had presented itself at Medtec LIVE to exhibit its ability to be a one-stop shop for medical device testing. The company's services covered electrical and functional safety testing, cyber security and software, EMC, and biocompatibility. The experts from TÜV SÜD were featured in the program of the online trade show and congress with various talks, a live hack and an elevator pitch
- In June 2020, Intertek announced the expansion of its personal protective equipment services, including precertification testing of N95 respirators to requirements set by the National Institute for Occupational Safety and Health (NIOSH). The new services are the result of the successful accreditation to NIOSH Standard Test Protocols, under ISO 17025. With these new services, Intertek also expands upon its solutions and resources to support customers and the global community during the COVID-19 pandemic.
Major Players
Data Bridge Market Research recognizes the following companies as the market players in medical device testing market: SGS (Switzerland), Eurofins Scientific (U.K.), Bureau Veritas (U.K.), Intertek (U.K.), TÜV SÜD (U.K.), DEKRA (U.K.), BSI (U.K.), TÜV Rheinland (U.K.), Elements Material Technology (U.K.), Envigo (U.S.), Avomeen Analytical Services (U.S.), Gateway Analytical (U.S.), Medistri SA (Switzerland), North American Science Associates (U.S.), Pace Analytical Services (U.S.), Wuxi Apptec (China), Toxikon (U.S.), Charles River Laboratories (U.S.), Medical Device Testing services (U.S.), Source Bioscience (U.K.), NSF International (U.S.), BDC laboratories (U.S.) and Surpass (U.S.).
The global medical device testing market is expected to reach USD 8,423.14 million by 2029 from USD 3,832.27 million in 2021, growing at a CAGR of 10.8% in the forecast period of 2022 to 2029. The rising need for global verification and validation of medical devices has enhanced the market's growth. The rising healthcare expenditure for better health services also contributes to the market's growth. Some of the major market players focus on various service launches and approvals during this crucial period.
Investing in this study would grant you access to valuable information, including:
- Comprehensive coverage of the Industry, both globally and broken down by region.
- Regional-level breakdowns of the market, including North America, Europe, Asia Pacific, South America, and the Middle East Africa.
- Country-specific market size splits for the most important countries with major market shares.
- Market share and revenue/sales data for the industry's leading players.
- Analysis of market trends such as emerging technologies, products, and start-ups, as well as PESTEL Analysis, SWOT Analysis, Porter's Five Forces, and more.
- Detailed market size data, including breakdowns by application/industry verticals.
- Projections and forecasts for the market's future growth and development.
Core Objective of the This Market:
- This Market Size and growth rate factors.
- Important changes in the future this Market.
- Top worldwide competitors of the Market.
- Scope and product outlook of This Market.
- Developing regions with potential growth in the future.
- Tough Challenges and risk faced in Market.
- Global top manufacturers profile and sales statistics.
Browse More Reports:
Europe Medical Device Testing Market
Asia-Pacific Medical Device Testing Market
Middle East and Africa Medical Device Testing Market
About Data Bridge Market Research:
An absolute way to forecast what future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavours to provide appropriate solutions to the complex business challenges and initiates an effortless decisionmaking process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475