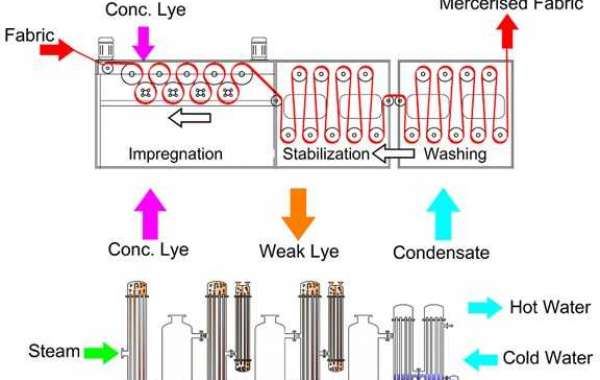
And mining industries. In these industries, caustic soda is commonly used for processes such as textile bleaching, pulp and paper production, water treatment, and alumina refining in the mining industry.
One of the key considerations in these industries is the concentration and recovery of caustic soda. This is important for several reasons:
Cost Efficiency: Caustic soda can be expensive, and recovering and reusing it can significantly reduce operating costs.
Environmental Impact: The production of caustic soda often involves the use of chlorine gas and can generate Caustic Soda Concentration Caustic Soda (Naoh) Recovery in the Textile Chlor Alkali Mining Industry waste by-products. Effective recovery and recycling of caustic soda can minimize environmental impact.
Process Efficiency: Maintaining the desired concentration of caustic soda is crucial for process efficiency and product quality. Some processes require specific concentrations of caustic soda for optimal results.
Here are some common methods for caustic soda concentration and recovery in these industries:
1. Evaporation:
This method involves heating a solution containing caustic soda to evaporate water, leaving behind a more concentrated caustic soda solution.
The vapor can be condensed and collected for reuse.
This method is widely used in industries where large volumes of water need to be removed.
2. Ion Exchange:
Ion exchange resins can be used to selectively remove water from a caustic soda solution.
The resin exchanges sodium ions for hydrogen ions in the caustic soda solution, effectively concentrating it.
Regeneration of the resin with a caustic soda solution allows for continuous operation.
3. Membrane Filtration:
Membrane processes like reverse osmosis or electrodialysis can be used to concentrate caustic soda solutions.
These methods use semipermeable membranes to separate water from the caustic soda, producing a more concentrated solution.
4. Crystallization:
Crystallization involves cooling a hot, concentrated caustic soda solution to allow crystals of sodium hydroxide to form.
The crystals can be separated from the remaining solution, which is typically less concentrated.
The crystals can be dissolved in water to produce a more concentrated caustic soda solution.
5. Electrodialysis:
Electrodialysis utilizes ion-exchange membranes and an electrical field to separate ions from a caustic soda solution.
This can lead to the concentration of caustic soda while leaving behind a less concentrated solution.
The choice of method depends on factors such as the initial concentration of caustic soda, the required final concentration, the volume of solution to be processed, energy availability, and cost considerations.
In addition to concentration methods, it's also important to consider safety measures and regulatory compliance when handling and recovering caustic soda, as it is a highly caustic and hazardous substance. Proper equipment, storage, and handling procedures should be in place to ensure safety and environmental responsibility.