Roots Analysis is pleased to announce the publication of its recent study, titled, “Antibody Drug Conjugate Market (7th Edition) Market, 2023-2035.”
The market report presents an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this domain, across different geographies. Amongst other elements, the report includes:
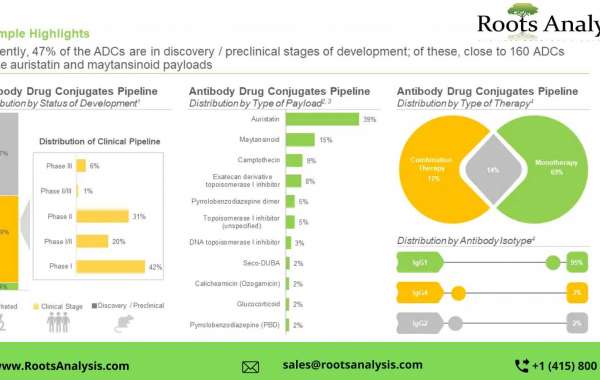
- A detailed assessment of the market landscape of close to 400 antibody drug conjugates / ADC therapeutics that are either approved or being evaluated in different stages of development
- An insightful competitiveness analysis of biological targets, featuring insightful pictorial summaries and representations.
- Elaborate profiles of leading antibody drug conjugate companies (shortlisted based on sales revenue of 2022) and their respective product portfolios.
- An in-depth analysis of completed, ongoing, and planned clinical studies of various antibody drug conjugates
- An insightful analysis, highlighting the key opinion leaders (KOLs) investigating clinical trials related to antibody drug conjugates
- An assessment of various therapeutics that are being evaluated in combination with antibody-drug conjugates.
- A detailed analysis of partnerships established by stakeholders engaged in this industry, since 2014
- An analysis of the various funding and investments made in the ADC domain, in the recent past
- An in-depth analysis of the various patents that have been filed / granted related to antibody drug conjugates
- A study of the various grants that have been awarded to research institutes engaged in conducting research related to antibody drug conjugates, since 2016
- An elaborate discussion on commercialization strategies adopted by various drug developers for their respective products
- An analysis of the key promotional strategies that have been adopted by the developers of marketed products
- An insightful success protocol analysis of recently approved ADC therapeutics, based on several relevant parameters
- An overview on of various conjugation and linker technologies along with their types that are presently being employed in the designing and development of antibody drug conjugates
- An overview of the studies conducted to better analyze non-clinical data and support first-in-human (FIH) dose selection in antibody drug conjugates
- An elaborate discussion on various factors that form the basis for the pricing of antibody drug conjugate products
- A case study on manufacturing of antibody drug conjugates, highlighting the key challenges, and a list of contract service providers that are involved in the ADC market.
- A case study on companies offering companion diagnostics that can potentially be used to make treatment related decisions involving antibody-drug conjugates
- A discussion on affiliated trends, key drivers, and challenges, under a comprehensive SWOT framework, which are likely to impact the industry’s evolution
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
- Target Disease Indication
- Acute Lymphoblastic Leukemia
- Breast Cancer
- B-Cell Lymphoma
- Cervical Cancer
- Gastric Cancer
- Lung Cancer
- Multiple Myeloma
- Renal Cancer
- Other Target Disease Indications
- Therapeutic Area
- Hematological Cancer
- Solid Tumor
- Linker
- Hydrazone (4-(4-Acetylphenoxy) Butanoic Acid (Acbut)
- Maleimide
- Maleimidocaproyl
- Succinimidyl-4-(N-Maleimidomethyl) Cyclohexane-1-Carboxylate
- Tetrapeptide-Based Linker
- Valine-Alanine
- Valine-Citrulline
- Other Linkers
- Payload
- DM1
- DM4
- Duocarmycin
- Monomethyl auristatin E (MMAE)
- Monomethyl auristatin F (MMAF)
- Ozogamicin
- SG3199
- SN-38 / Irinotecan
- Other Payloads
- Target Antigen
- BCMA (TNFRSF17 / BCM)
- CD19
- CD22
- CD30
- CD79b
- CEACAM5
- HER-2 (ERBB2)
- Nectin 4
- Tissue factor
- TROP-2
- Other Target Antigen
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
Key companies covered in the report
- ADC Therapeutics
- Astellas Pharma
- AstraZeneca
- Byondis
- Daiichi Sankyo
- Genentech
- Gilead Sciences
- ImmunoGen
- Pfizer
- RemeGen
To view more details on this report, click on the link:
https://www.rootsanalysis.com/reports/view_document/antibody-drug-conjugates-market/270.html
News article
Learn from experts: do you know about these emerging industry trends?
Investment Opportunities in Women’s Digital Health
Genotoxicity Testing: Unlocking the Future Safety Assessment Opportunities
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Roots Analysis Consulting - the preferred research partner for global firms
Contact:
Ben Johnson
+1 (415) 800 3415