Industry Analysis
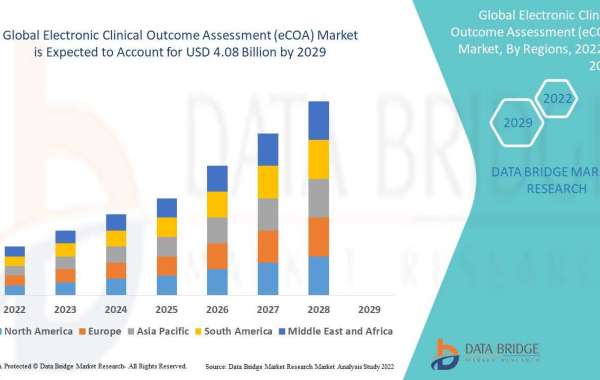
The state of a patient and the consequences and course of a disease are determined via electronic clinical outcome assessments (eCOA). The electronic clinical outcome assessments enable real-time visibility, which aids in enhancing protocol adherence, assuring patient safety and participation, and lowering trial risks. Data Bridge Market Research analyses that the electronic clinical outcome assessment (eCOA) market which was USD 1.22 billion in 2021, would rocket up to USD 4.08 billion by 2029, and is expected to undergo a CAGR of 16.30% during the forecast period 2022 to 2029. The market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Electronic Clinical Outcome Assessment (eCOA) market research report has put forth a bench-marking example for a vibrant market that explores several recommendations and practical growth strategies in relation to the market. The market report covers a wide spectrum of regions and also focuses on key regions that include North America, Europe, Asia Pacific, Middle East, South America, and the Middle East Africa (MEA). The chapter of the competitive landscape is presented well in the market research report and is analysed based on the tools such as Porter’s five forces analysis. The study throws light upon market attractiveness where all the segments are arranged based on the compound growth rate, size, and general attractiveness.
Get Sample Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-electronic-clinical-outcome-assessment-ecoa-market
Electronic Clinical Outcome Assessment (eCOA) Market Dynamics
Drivers
- Rising need for efficiency of clinical trials
The market for electronic clinical outcome assessment (eCOA) is being driven by clinical trials and the growing demand for integrated and automated processes. The solutions are essential for improving study data quality and complying with regulatory standards.
- Increasing number of clinical trials
The market is expected to rise due to the need to improve compliance, effectively capture and manage clinical information, cost reductions, and increase research and development (R RD) activities.
- Rising burden on pharmaceutical manufacturers
New medication development techniques are increasingly turning to electronic data capture rather than paper-based procedures to reduce total expenses. Data collecting using electronic clinical outcome assessments platforms improves the quality of data obtained, harmonises data gathering practises, and provides considerable value to its users, such as data analysis. Electronic-based data collecting and analysis services overcome all of the drawbacks of paper-based records while also increasing patient compliance. They also reduce the expense of site monitoring and eliminate the danger of data fluctuation. These solutions provide streamlined information, which helps to improve data quality by gathering data in a structured manner. In the near years, the aforementioned advantages of adopting electronic clinical outcome assessments are projected to stimulate product demand.
Opportunities
The market is growing due to medical research specialists' increasing use of electronic clinical outcome assessments. As the number of research projects grows, so does the need for a centralised data collection system that may help increase patient participation. Over the foreseeable period, this is expected to increase market demand. During the pandemic, the use of electronic patient reported outcomes (ePROs) has developed as a useful method for collecting and sharing essential trial data and using additional embedded tools like alerts and reminders to weave the study into patients daily life. Technological advances including Alexa-style tools, reward features, and gamification are expected to improve electronic clinical outcome assessment (eCOA) solutions in the future, resulting in market growth.
Restraints/Challenges
Data privacy issues and expensive implementation costs, on the other hand, are limiting the market's expansion. In addition, in the forecast period of 2022-2029, the market is expected to be challenged by a lack of advantageous reimbursement scenarios in developing economies, high custom duty imposed on medical equipment, and a lack of sufficient infrastructure in low- and middle-income nations.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-market
Industry Segmentation and Size
The electronic clinical outcome assessment (eCOA) market is segmented on the basis of product, delivery mode, approach, platform and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- On-Premise Solutions
- Cloud Based Solutions
- Web Based Solutions
Delivery Mode
- Web-hosted
- Cloud-based
Approach
- Patient-reported Outcome (PRO)
- Clinician-reported Outcome (ClinRO)
- Observer-reported Outcome (ObsRO)
- Performance Outcome (PerfO)
Platform
- Contract Research Organizations
- Pharmaceutical and Biopharmaceutical Companies
- Medical Device Manufacturers
- Hospitals and Clinical Laboratories
- Consulting Service Companies
- Research and Academia
- Others
End-User
- Pharmaceutical and Biopharmaceutical Companies
- Contract Research Organizations
- Other End-Users
Market Country Level Analysis
- The countries covered in the electronic clinical outcome assessment (eCOA) market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Industry Share Analysis
Some of the major players operating in the electronic clinical outcome assessment (eCOA) market are:
- IBM Corporation (U.S)
- IQVIA (U.S)
- Medidata Solutions, Inc. (U.S)
- Clario (U.S)
- ArisGlobal (U.S)
- Signant Health (U.S)
- TransPerfect (U.S)
- Cloudbyz (U.S)
- Climedo Health GmbH (Germany)
- ClinCapture (U.S)
- Oracle Corporation (U.S)
- Paraxel International Corporation (U.S)
- eClinical Solutions LLC (U.S)
- OmniComm Systems, Inc. (U.S)
- CRF Health (U.S)
- European Round Table (U.S)
Electronic Clinical Outcome Assessment (eCOA) Market Report Answers the Following Questions:
- What is the current size of the Electronic Clinical Outcome Assessment (eCOA) Market?
- What are the key drivers and challenges for the Electronic Clinical Outcome Assessment (eCOA) Market?
- What are the different types of Electronic Clinical Outcome Assessment (eCOA) Market?
- What are the leading companies operating in the Electronic Clinical Outcome Assessment (eCOA) Market?
- What is the future outlook for the Electronic Clinical Outcome Assessment (eCOA) Market?
Browse Related Reports@
Electronic Clinical Outcome Assessment (eCOA) Market
Capillary Blood Collection Devices Market
About Us: Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact: Data Bridge Market Research
Tel: +1-888-387-2818
Email: Sopan.gedam@databridgemarketresearch.com