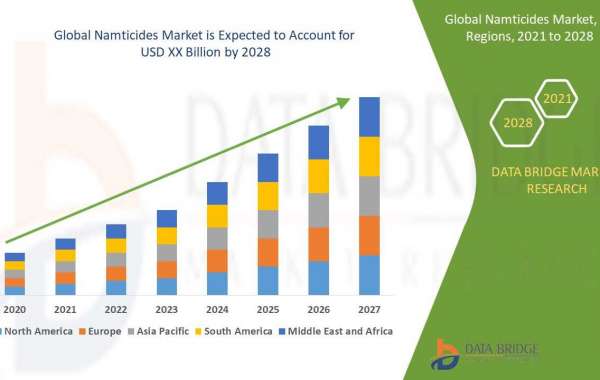
How Does Real-World Evidence Impact Pharmaceutical Drug Development?
Real-world evidence (RWE) is transforming pharmaceutical drug development by providing insights into how drugs perform outside of controlled clinical trials. By leveraging real-world data (RWD) from sources like electronic health records (EHRs), insurance claims, and patient registries, pharmaceutical companies can understand drug efficacy, safety, and long-term outcomes in diverse populations.
For example, RWE is crucial in post-market surveillance, where pharmaceutical companies track adverse drug reactions in broader patient groups than those used in clinical trials. Companies like Pfizer and Roche are using real-world data to accelerate drug approval processes and personalize treatments. One innovation in this area is machine learning models that analyze vast datasets to predict patient responses to new therapies.
2. What are the Regulatory Challenges of Using Real-World Evidence in Healthcare?
Despite its potential, there are regulatory challenges to the widespread adoption of RWE in healthcare. Regulatory bodies, such as the FDA and the European Medicines Agency (EMA), are cautious about the quality and reliability of real-world data. Concerns about data privacy, standardization, and the representativeness of datasets create hurdles.
For instance, ensuring data from different healthcare systems are comparable remains a challenge. Top pharmaceutical companies like AstraZeneca and Novartis are investing in technologies that standardize real-world data and meet regulatory requirements. Blockchain technology is being explored to enhance data security and integrity, ensuring patient data privacy while enabling broader use of RWE.
3. How Does Real-World Evidence Improve Patient Outcomes?
Real-world evidence provides critical insights into patient outcomes by analyzing data from everyday healthcare settings. By examining patient responses to treatments in a naturalistic environment, healthcare providers can tailor interventions to specific patient groups.
For example, in oncology, RWE is used to identify which cancer treatments work best for patients with specific genetic markers. This personalized approach improves treatment effectiveness and patient survival rates. Companies like Bristol Myers Squibb and Johnson & Johnson are pioneering the use of artificial intelligence (AI) and genomics to gather real-world evidence, ensuring that patients receive the most appropriate and effective treatments based on their unique health profiles.
4. Can Real-World Evidence Replace Randomized Controlled Trials?
While real-world evidence is valuable, it is unlikely to fully replace randomized controlled trials (RCTs) in the near future. RCTs remain the gold standard for proving the efficacy of new treatments under controlled conditions. However, RWE complements RCTs by providing additional data on long-term safety and effectiveness in larger, more diverse patient populations.
Innovative technologies like cloud-based platforms and wearables are being used by companies such as IQVIA and Medtronic to collect real-time patient data, bridging the gap between traditional clinical trials and real-world studies. These innovations allow for continuous monitoring of patient outcomes, offering a more comprehensive understanding of treatment impacts over time.
| For more info. | Market Research | Related Report | AI in Medical Diagnostics Market |
| Ovarian Cancer Treatment Drugs Market | |||
| Sleep Apnea Implants Market |