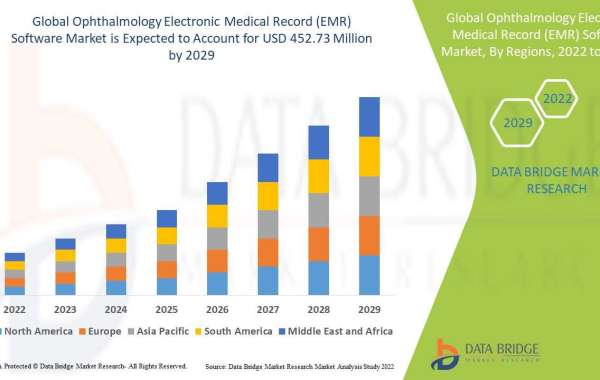
What Are the Latest Trends in the Point of Care Testing Market for 2024?
The Point of Care (POC) testing market is witnessing significant growth, projected to reach USD 50 billion by 2024, driven by the increasing demand for rapid diagnostic results and the shift towards decentralized healthcare. Key trends shaping the market include:
Integration of Artificial Intelligence (AI): AI technologies are being integrated into POC devices to enhance diagnostic accuracy and speed. Companies like Abbott and Siemens Healthineers are leveraging AI to analyze complex data quickly.
Wearable Diagnostic Devices: Innovations in wearable technology are enabling real-time health monitoring, with companies like Dexcom and Philips leading the way in developing glucose monitoring devices and remote patient monitoring solutions.
Telehealth Expansion: The rise of telehealth has accelerated the adoption of POC testing, allowing patients to receive immediate results and consultation. This trend is evident in the offerings from companies like LabCorp and Quest Diagnostics, which have expanded their POC testing capabilities to support remote healthcare.
Regulatory Support: Regulatory bodies are streamlining the approval processes for POC devices, encouraging innovation. The FDA has introduced programs to expedite the review of POC diagnostics, enabling faster access to essential testing.
What Are the Recent Advancements in Point of Care Diagnostics Technology?
Recent advancements in point of care diagnostics technology are transforming healthcare delivery. Key innovations include:
Microfluidics: This technology allows for the manipulation of small volumes of fluids, enabling rapid and accurate diagnostics. Companies like Cepheid are utilizing microfluidic devices for fast infectious disease testing.
Molecular Testing: The use of molecular diagnostics, particularly in detecting viruses and bacteria, has seen significant advancements. Companies like Roche Diagnostics are leading the way with their rapid molecular testing platforms.
Portable Imaging Technologies: Innovations in portable imaging, such as handheld ultrasound devices developed by GE Healthcare, are enhancing the ability of healthcare providers to conduct diagnostics at the point of care.
Rapid Test Kits: Development of easy-to-use test kits for various conditions, including COVID-19 and diabetes, is gaining traction. Companies like Abbott have introduced rapid antigen tests that provide results within minutes.
What Are the Key Regulatory Challenges Faced by Point of Care Testing Products?
While the POC testing market is booming, it is not without challenges. Key regulatory hurdles include:
Approval Delays: Although regulatory bodies are streamlining processes, delays in approvals can still impede market entry for new products. Companies must navigate complex regulations to ensure compliance.
Quality Control Standards: Ensuring the reliability and accuracy of POC tests is crucial. Regulatory agencies require rigorous validation and quality control measures, which can be resource-intensive for manufacturers.
Post-Market Surveillance: Companies must conduct ongoing monitoring and reporting of the performance of their POC tests post-launch, leading to additional costs and operational challenges.
Evolving Regulations: Regulatory standards for diagnostics are continuously evolving, necessitating that companies stay updated on changes to maintain compliance and avoid penalties.
What Market Opportunities Exist for Companies in the Point of Care Diagnostics Sector?
The point of care diagnostics market presents numerous opportunities for companies looking to innovate and expand their offerings. Key opportunities include:
Emerging Markets: There is a growing demand for POC testing solutions in emerging markets due to increased healthcare accessibility and a focus on preventive care. Companies like Mylab Discovery Solutions are successfully tapping into these markets.
Collaboration with Healthcare Providers: Partnering with hospitals and clinics can enhance product visibility and adoption. Companies like Siemens Healthineers are collaborating with healthcare providers to implement POC testing solutions effectively.
Expansion of Disease Testing Portfolio: Companies can expand their testing portfolios to include a wider range of diseases, including chronic and infectious diseases. For instance, companies like Becton, Dickinson and Company are continuously innovating to offer comprehensive diagnostic solutions.
Investment in R&D: Focusing on research and development to create next-generation POC devices can yield substantial returns. Investment in R&D can help companies stay ahead of competitors and meet evolving healthcare needs.
Conclusion
The Point of Care Diagnostics/Testing Market is poised for significant growth, driven by technological advancements and an evolving healthcare landscape. By understanding the latest trends, innovations, regulatory challenges, and market opportunities, stakeholders can position themselves strategically in this dynamic field.
Top Companies Innovating in Point of Care Testing:
- Abbott
- Siemens Healthineers
- Roche Diagnostics
- Cepheid
- LabCorp
- Quest Diagnostics
- GE Healthcare
- Mylab Discovery Solutions
- Becton, Dickinson and Company
| For more info. | Market Research | Related Report | brazil pharmaceutical industry Market |
| us mental health services Market | |||
| software as a medical device Market |