What is Bioluminescence Resonance Energy Transfer?
Bioluminescence Resonance Energy Transfer (BRET) assay is a powerful technique used to study protein-protein interactions in live cells. It is based on the principle of energy transfer between a bioluminescent donor protein and a fluorescent acceptor protein. This assay has gained widespread popularity due to its ability to detect weak or transient interactions that are difficult to observe using other techniques. The BRET assay has been used in various biological systems, including signaling pathways, transcriptional regulation, protein folding, and virus-host interactions.
Principle of BRET Assay
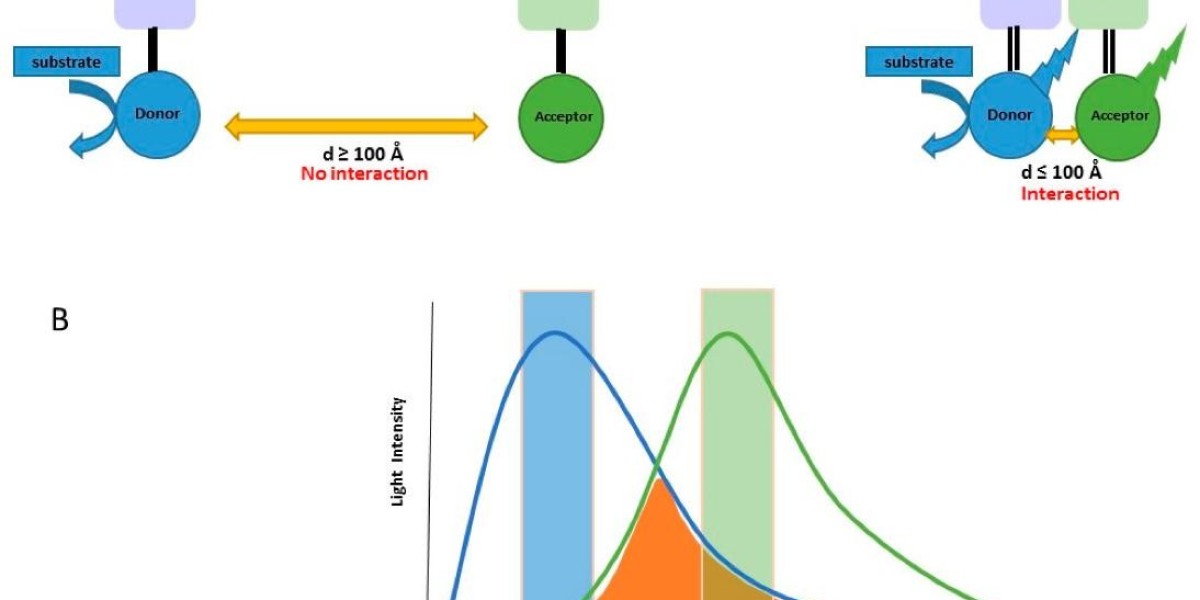
The BRET assay is based on the principle of resonance energy transfer, a non-radiative process that occurs between two fluorophores in close proximity. In the BRET assay, a bioluminescent protein (e.g., Renilla luciferase) acts as the donor and a fluorescent protein (e.g., GFP or YFP) acts as the acceptor. When the donor and acceptor are in close proximity (typically <10 nm), energy is transferred from the excited donor to the acceptor through RET, resulting in the emission of fluorescence from the acceptor.
The efficiency of energy transfer depends on several factors, including the spectral overlap between the donor emission and acceptor excitation spectra, the distance between the donor and acceptor, and the relative orientation of their transition dipoles. Therefore, the BRET assay is highly sensitive to changes in the distance and orientation of the donor and acceptor, making it an ideal tool for studying protein-protein interactions.
How Does BRET Work?
The BRET assay typically involves the expression of two fusion proteins: a donor protein fused to one of the interacting proteins and an acceptor protein fused to the other interacting protein. The donor and acceptor fusion proteins are expressed in the same cell, and the cells are stimulated to induce protein-protein interaction. The donor protein is then excited with a substrate (such as coelenterazine) to produce bioluminescence, which in turn excites the acceptor protein through RET. The fluorescence emitted by the acceptor protein is then detected using a fluorescence detector.
One of the key advantages of the BRET assay is that it can be performed in live cells, allowing the study of protein-protein interactions in their native environment. Moreover, the BRET assay is highly sensitive and can detect weak or transient interactions that are difficult to observe using other techniques.

Bioluminescence Resonance Energy Transfer method (El Khamlichi et al., 2019).
Applications of BRET Assay
The BRET assay has been widely used to study protein-protein interactions in a variety of biological systems, including signaling pathways, transcriptional regulation, protein folding, and virus-host interactions. For example, the BRET assay has been used to study the interaction between G protein-coupled receptors (GPCRs) and their downstream effectors, such as G proteins and arrestins. In addition, the BRET assay has been used to study the regulation of protein-protein interactions by small molecules, such as agonists and antagonists.
The BRET assay has also been used to study the structure and function of proteins, including protein folding and conformational changes. For example, the BRET assay has been used to study the conformational changes of the prion protein, which is implicated in the development of prion diseases such as Creutzfeldt-Jakob disease.
Another application of the BRET assay is in drug discovery, where it is used to screen for compounds that modulate protein-protein interactions. The BRET assay can be used to screen large libraries of compounds to identify molecules that bind to a specific protein and disrupt its interaction with another protein. This approach has been used to identify compounds that inhibit the interaction between p53 and MDM2, a key regulator of the tumor suppressor p53, and to identify inhibitors of the HIV-1 protein-protein interactions.
Moreover, the BRET assay has also been used to study virus-host interactions, including the interactions between viral proteins and host cell proteins. For example, the BRET assay has been used to study the interactions between the hepatitis C virus (HCV) NS5A protein and host cell proteins involved in the innate immune response.

The BRET assay has been developed to study protein–protein interactions (Siddiqui et al., 2013).
Limitations and Considerations
Although the BRET assay has several advantages over other techniques for studying protein-protein interactions, it also has some limitations and considerations. One limitation is that the BRET assay requires the expression of fusion proteins, which can potentially affect the localization, stability, and activity of the interacting proteins. Therefore, careful controls and validation are necessary to ensure that the observed interactions are biologically relevant.
Another consideration is the choice of donor and acceptor proteins, which can affect the sensitivity and specificity of the assay. The choice of donor and acceptor proteins should be based on their spectral properties, expression levels, and potential interactions with other proteins in the system.
References
- El Khamlichi, Chayma, et al. "Bioluminescence resonance energy transfer as a method to study protein-protein interactions: application to G protein coupled receptor biology." Molecules 24.3 (2019): 537.
- Siddiqui, Sana, et al. "BRET biosensor analysis of receptor tyrosine kinase functionality." Frontiers in endocrinology 4 (2013): 46.
Read More: crosslinking protein interaction